
Let's begin by discussing the vapor pressure of a pure substance and how it varies with temperature.
#Arrange these compounds by their expected vapor pressure. how to
In order to understand how to take advantage of these processes in purifying organic materials, we first need to learn how pure compounds behave when they are vaporized or sublimed. Both vaporization and sublimation are processes that can be used to purify compounds.
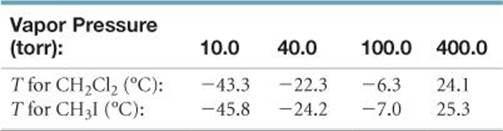
This is a consequence of the process of sublimation. Many of you may have noticed that even on a day in which the temperature stays below freezing, the volume of snow and ice will appear to decrease, particularly from dark pavements on the streets. They simply vaporize directly into the atmosphere. Solid substances are not characterized by a similar phenomena as boiling. If the external pressure is atmospheric pressure, the temperature at which a pure substance boils is called the normal boiling point. When the vapor pressure of a liquid substance reaches the external pressure, the substance is observed to boil. The larger the vapor pressure, the greater the tendency to escape. Vapor pressure is a measure of the tendency of a condensed substance to escape the condensed phase. The vapor pressure of a pure substance is the pressure exerted by the substance against the external pressure which is usually atmospheric pressure. All substances regardless of whether they are liquids or solids are characterized by a vapor pressure. The wind facilitates the evaporation process and you supply some of the heat that is required. This is why even on a hot day at the beach, if there is a strong breeze blowing, it may feel cool or cold after you come out of the water. For a liquid, this process is called vaporization and for a solid it is called sublimation. Let us begin by describing the process by which a substance is transformed from the condensed phase to the gas phase. Many of these are terms that you are familiar with but the exact definitions may not be known to you. However, before we begin a discussion of distillation, it would probably be beneficial to define the terms that describe the process and related properties. From 0☌ to 80☌, the vapor pressure of water increases by 46.73 kPa, while it increases by 53.99 kPa in only a span of twenty degrees from 80☌ to 100☌.Distillation is an important commercial process that is used in the purification of a large variety of materials. Notice that the temperature dependence of the vapor pressure is not linear. Vapor Pressure (in kPa) of Three Liquids at Different Temperatures The Table below shows the temperature dependence of the vapor pressure of three liquids. The greater number of vapor molecules strike the container walls more frequently, resulting in an increase in pressure.
When the liquid in a closed container is heated, more molecules escape the liquid phase and evaporate. Vapor pressure is dependent upon temperature. The vapor pressure of water at 20☌ is only 2.33 kPa, far less than that of diethyl ether. Water is a polar liquid whose molecules are attracted to one another by relatively strong hydrogen bonding. For example, diethyl ether is a nonpolar liquid with weak dispersion forces. A liquid with stronger intermolecular forces does not evaporate easily and thus has a lower vapor pressure. A liquid with weak intermolecular forces evaporates more easily and has a high vapor pressure. Vapor pressure is a property of a liquid based on the strength of its intermolecular forces.
The vapor pressure is a measure of the presure (force per unit area) exerted by a gas above a liquid in a sealed container. The forward direction represents the evaporation process, while the reverse direction represents the condensation process.īecause they cannot escape the container, the vapor molecules above the surface of the liquid exert a pressure on the walls of the container. Equilibrium between liquid phase and vapor phase.Ī dynamic equilibrium can be illustrated by an equation with a double arrow, meaning that the reaction is occurring in both directions and at the same rate.


 0 kommentar(er)
0 kommentar(er)
